Shining a UV light to separate rare earth metals (Day 354)

16th May 2015
There are 17 rare earth metals in the periodic table, but they are not 'rare' because of a lack of abundance – they are rare because they are usually found dispersed in small amounts.

These rare earth metals find use in many modern day applications ranging from healthcare and electronics, to computers and advanced transportation. Two rare earth metals that are particularly useful in sustainable technology and high-tech applications – europium and yttrium.
Europium and yttrium are difficult to mine but they can be recycled and recovered from another source - red lamp phosphor (a powder used in fluorescent lamps and low energy light bulbs).
Chemical engineers from the University of Leuven (KE Leuven), Belgium, have developed a method that recycles the red lamp phosphor as well as separates the rare earth metals, europium and yttrium, from a mixture using UV light.

Professor Tom Van Gerven, from the department of chemical engineering at KE Leuven, explained the conventional process of separating rare earth metals: "The traditional method dissolves europium and yttrium in aqueous acid.
"An extractant and a solvent are then added to the aqueous liquid, leading to two separate layers or 'phases': an aqueous layer containing the rare earth metals and a solvent layer with the extractant. When the two layers come into contact, one of the two rare earth metals is extracted to the solvent, while the other rare earth metal remains in the aqueous layer.
"But this process leaves much to be desired in terms of efficiency and purity: it needs to be repeated dozens of times to recover a high percentage of a particular rare earth metal, and there will still be traces of yttrium in the europium-containing liquid and vice versa."
So instead of using a solvent, Tom and the team of chemical engineers working in collaboration with chemists, also from KE Leuven, used UV light to recover europium from the liquid mixture.
PhD student, Bart Van den Bogaert, explains: "The UV light influences the electrically charged ions. Both europium and yttrium have three positive charges per ion. When we shine UV light upon the solution of europium and yttrium, we add energy to the system.
"As a result, one positive charge per europium ion is neutralised. When we add sulphate, only the europium reacts with it. The result is a precipitate that can easily be filtered, while the yttrium remains in the solution."
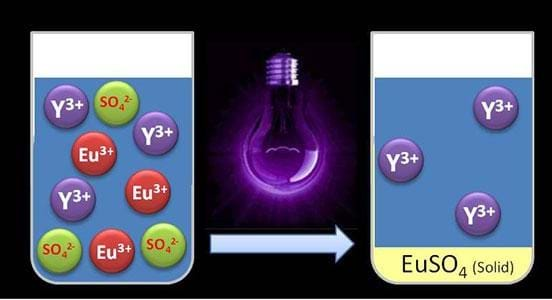
Compared with the more conventional methods, using UV light to separate the rare earth metals gives a higher separation efficiency, where more than 95 per cent of europium is recovered. Additionally, the UV light method does not produce any harmful chemicals as a by-product.
The team of researchers published their findings in the journal Green Chemistry titled: 'Photochemical recycling of europium from Eu/Y mixtures in red lamp phosphor waste streams'.
This work could bring about new opportunities for recycling fluorescent lamps and low-energy light bulbs. So I commend the team for their research efforts and wish them well for the next stage, which is to improve the separation efficiency even further.
Do you work with rare earth elements, if so, get in touch and share your research.